Background
Tuberculosis
(TB), a multisystemic disease with myriad presentations and
manifestations, is the most common cause of infectious disease–related
mortality worldwide. The World Health Organization (WHO) has estimated
that 2 billion people have latent TB and that globally, in 2009, the
disease killed 1.7 million people.[1] New TB treatments are being developed,[2] and new TB vaccines are under investigation. (See Epidemiology and Treatment and Management, below.)[3]
Although TB rates are decreasing in the United States, the disease is becoming more common in many parts of the world. In addition, the prevalence of drug-resistant TB is also increasing worldwide. Co-infection with the human immunodeficiency virus (HIV) has been an important factor in the emergence and spread of resistance. (See Treatment of Multidrug-Resistant TB, below.)[4]
TB is an ancient disease. Signs of skeletal TB (Pott disease) were evident in Europe from Neolithic times (8000 BCE), in ancient Egypt (1000 BCE), and in the pre-Columbian New World. TB was recognized as a contagious disease by the time of Hippocrates (400 BCE), when it was termed "phthisis" (Greek from phthinein, to waste away).
Mycobacterium tuberculosis, a tubercle bacillus, is the causative agent of TB. It belongs to a group of closely related organisms—including M africanum, M bovis, and M microti —in the M tuberculosis complex. Robert Koch discovered and isolated M tuberculosis in 1882. (See Etiology, below.) An image of the bacterium is seen below.
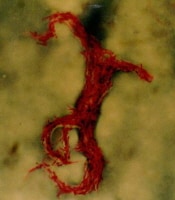 Acid-fast bacillus smear showing characteristic cording in Mycobacterium tuberculosis. World
incidence of TB increased with population density and urban
development, so that by the Industrial Revolution in Europe (1750), it
was responsible for more than 25% of adult deaths. Indeed, in the early
20th century, TB was the leading cause of death in the United States.
(See Etiology and Epidemiology, below.)
Acid-fast bacillus smear showing characteristic cording in Mycobacterium tuberculosis. World
incidence of TB increased with population density and urban
development, so that by the Industrial Revolution in Europe (1750), it
was responsible for more than 25% of adult deaths. Indeed, in the early
20th century, TB was the leading cause of death in the United States.
(See Etiology and Epidemiology, below.)
The US Centers for Disease Control and Prevention (CDC) has been recording detailed epidemiologic information on tuberculosis (TB) since 1953. The incidence of TB has been declining since the early 20th century because of various factors, including basic infection-control practices (isolation). Beginning in 1985, a resurgence of TB was noted. The increase was observed primarily in ethnic minorities and especially in persons infected with HIV. TB control programs were revamped and strengthened across the United States. (See Epidemiology.)
As an AIDS (acquired immunodeficiency syndrome)-related opportunistic infection, TB is associated with HIV infections, with dual infections being frequently noted. Globally, coinfection with HIV is highest in South Africa, India, and Nigeria.
Persons with AIDS are 20-40 times more likely than immunocompetent persons to develop active TB.[5] Correspondingly, TB is the leading cause of mortality among persons infected with HIV.[6]
Worldwide, TB is most common in Africa, the West Pacific, and Eastern Europe. These regions are plagued with factors that contribute to the spread of TB, including the presence of limited resources, HIV infection, and multidrug-resistant (MDR) TB. Consequently, although international public health efforts have put a huge curb on the rate of increase in TB, these regions account for the continued increase in global TB. (See Epidemiology.)
Multiple factors contribute to the drug resistance of M tuberculosis, including incomplete and inadequate treatment or adherence to treatment, logistical issues, virulence of the organism, multidrug transporters, host genetic factors, and HIV infection.
According to WHO, the prevalence of MDR-TB has been 1.1% in newly diagnosed patients; it is reportedly even higher in patients who have previously received anti-TB treatment (7%).
MDR-TB and XDR-TB are becoming increasingly significant.[7] Genotype studies have shown that between 63% and 75% of XDR-TB cases progress through acquisition of resistance.[8]
According to the US National TB Surveillance System (NTSS), between 1993 and 2006 a total of 49 cases (3% of evaluable MDR-TB cases) met the revised case definition for XDR-TB. The largest number of XDR-TB cases was found in New York City and California.
The success rate of treatment with standard short-course chemotherapy (SCC) is less than 60% in patients with MDR-TB, compared with a success rate of more than 85% in patients with drug-susceptible TB.
(MDR-TB and XDR-TB not only produce fulminant and fatal disease among patients infected with HIV [time from TB exposure to death averages 2-7 mo] but are also highly infectious, with conversion rates of as much as 50% in exposed health care workers.)
Out of these goals were born major TB surveillance programs and the concept of directly observed therapy (DOT), which requires a third party to witness compliance with pharmacotherapy. With worldwide efforts, global detection of smear-positive cases rose from 11% (1991) to 45% (2003), with 71-89% of those cases undergoing complete treatment.
A large percentage of ED patients are at increased risk for having active TB, including homeless/shelter-dwelling patients, travelers from endemic areas, immunocompromised patients, health care workers, and incarcerated patients. Therefore, emergency physicians must consider the management and treatment of TB as a critical public health measure in the prevention of a new epidemic.[11]
For high-risk cases, prehospital workers can assist in identifying household contacts who may also be infected or who may be at high risk of becoming infected.
Prehospital workers should be aware that any case of active TB in a young child indicates disease in 1 or more adults with close contact, usually within the same household. TB in a child is a sentinel event indicating recent transmission.
Most often, patients will complain of blurry vision that may or may not be associated with pain and red eye. In the rare case of orbital disease, proptosis, double vision, or extraocular muscle motility restriction may be the presenting complaint. Preseptal cellulitis in children with spontaneous draining fistula may also occur. In cases of both pulmonary and extrapulmonary TB, there may be ocular findings without ocular complaints.
In patients with confirmed active pulmonary or active nonocular extrapulmonary TB, ocular incidence ranges from 1.4-5.74%. In HIV patients, the incidence of ocular TB may be higher, with a reported prevalence of from 2.8-11.4%.
Although TB rates are decreasing in the United States, the disease is becoming more common in many parts of the world. In addition, the prevalence of drug-resistant TB is also increasing worldwide. Co-infection with the human immunodeficiency virus (HIV) has been an important factor in the emergence and spread of resistance. (See Treatment of Multidrug-Resistant TB, below.)[4]
TB is an ancient disease. Signs of skeletal TB (Pott disease) were evident in Europe from Neolithic times (8000 BCE), in ancient Egypt (1000 BCE), and in the pre-Columbian New World. TB was recognized as a contagious disease by the time of Hippocrates (400 BCE), when it was termed "phthisis" (Greek from phthinein, to waste away).
Mycobacterium tuberculosis, a tubercle bacillus, is the causative agent of TB. It belongs to a group of closely related organisms—including M africanum, M bovis, and M microti —in the M tuberculosis complex. Robert Koch discovered and isolated M tuberculosis in 1882. (See Etiology, below.) An image of the bacterium is seen below.
 Acid-fast bacillus smear showing characteristic cording in Mycobacterium tuberculosis. World
incidence of TB increased with population density and urban
development, so that by the Industrial Revolution in Europe (1750), it
was responsible for more than 25% of adult deaths. Indeed, in the early
20th century, TB was the leading cause of death in the United States.
(See Etiology and Epidemiology, below.)
Acid-fast bacillus smear showing characteristic cording in Mycobacterium tuberculosis. World
incidence of TB increased with population density and urban
development, so that by the Industrial Revolution in Europe (1750), it
was responsible for more than 25% of adult deaths. Indeed, in the early
20th century, TB was the leading cause of death in the United States.
(See Etiology and Epidemiology, below.) The US Centers for Disease Control and Prevention (CDC) has been recording detailed epidemiologic information on tuberculosis (TB) since 1953. The incidence of TB has been declining since the early 20th century because of various factors, including basic infection-control practices (isolation). Beginning in 1985, a resurgence of TB was noted. The increase was observed primarily in ethnic minorities and especially in persons infected with HIV. TB control programs were revamped and strengthened across the United States. (See Epidemiology.)
As an AIDS (acquired immunodeficiency syndrome)-related opportunistic infection, TB is associated with HIV infections, with dual infections being frequently noted. Globally, coinfection with HIV is highest in South Africa, India, and Nigeria.
Persons with AIDS are 20-40 times more likely than immunocompetent persons to develop active TB.[5] Correspondingly, TB is the leading cause of mortality among persons infected with HIV.[6]
Worldwide, TB is most common in Africa, the West Pacific, and Eastern Europe. These regions are plagued with factors that contribute to the spread of TB, including the presence of limited resources, HIV infection, and multidrug-resistant (MDR) TB. Consequently, although international public health efforts have put a huge curb on the rate of increase in TB, these regions account for the continued increase in global TB. (See Epidemiology.)
Drug-resistant TB
MDR-TB is defined as resistance to the 2 most effective first-line drugs, isoniazid and rifampin.[6] Another type of resistant TB, called extensively drug-resistant TB (XDR-TB), is resistant to isoniazid, rifampin, and second-line drugs used to treat MDR-TB. Mortality rates for patients with XDR-TB are similar to those of patients from the preantibiotic era. (Approximately 1 in 13 M tuberculosis isolates currently shows a form of drug resistance.)[6]Multiple factors contribute to the drug resistance of M tuberculosis, including incomplete and inadequate treatment or adherence to treatment, logistical issues, virulence of the organism, multidrug transporters, host genetic factors, and HIV infection.
According to WHO, the prevalence of MDR-TB has been 1.1% in newly diagnosed patients; it is reportedly even higher in patients who have previously received anti-TB treatment (7%).
MDR-TB and XDR-TB are becoming increasingly significant.[7] Genotype studies have shown that between 63% and 75% of XDR-TB cases progress through acquisition of resistance.[8]
According to the US National TB Surveillance System (NTSS), between 1993 and 2006 a total of 49 cases (3% of evaluable MDR-TB cases) met the revised case definition for XDR-TB. The largest number of XDR-TB cases was found in New York City and California.
The success rate of treatment with standard short-course chemotherapy (SCC) is less than 60% in patients with MDR-TB, compared with a success rate of more than 85% in patients with drug-susceptible TB.
(MDR-TB and XDR-TB not only produce fulminant and fatal disease among patients infected with HIV [time from TB exposure to death averages 2-7 mo] but are also highly infectious, with conversion rates of as much as 50% in exposed health care workers.)
Global surveillance and treatment of TB
As previously stated, multidrug resistance has arisen from poor compliance with TB therapies , resulting in difficulties in controlling the disease. Consequently, a threat of global pandemic occurred in the late 1980s and early 1990s. Reacting to these signals, the World Health Organization developed a plan to try to identify 70% of the world's cases of TB and to completely treat at least 85% of these cases by the year 2000.Out of these goals were born major TB surveillance programs and the concept of directly observed therapy (DOT), which requires a third party to witness compliance with pharmacotherapy. With worldwide efforts, global detection of smear-positive cases rose from 11% (1991) to 45% (2003), with 71-89% of those cases undergoing complete treatment.
Approach to TB in the emergency department
Despite the importance of early isolation of patients with active TB, a standardized triage protocol with acceptable sensitivities has yet to be developed.[9] Moran et al demonstrated that among patients with active TB in the emergency department (ED), TB was often unsuspected, and isolation measures were not used.[10] The difficulty in establishing such a protocol only highlights the importance of the emergency physician’s role in the prompt identification and isolation of active TB.A large percentage of ED patients are at increased risk for having active TB, including homeless/shelter-dwelling patients, travelers from endemic areas, immunocompromised patients, health care workers, and incarcerated patients. Therefore, emergency physicians must consider the management and treatment of TB as a critical public health measure in the prevention of a new epidemic.[11]
For high-risk cases, prehospital workers can assist in identifying household contacts who may also be infected or who may be at high risk of becoming infected.
Prehospital workers should be aware that any case of active TB in a young child indicates disease in 1 or more adults with close contact, usually within the same household. TB in a child is a sentinel event indicating recent transmission.
Extrapulmonary involvement in TB
Extrapulmonary involvement occurs in one fifth of all TB cases; 60% of patients with extrapulmonary manifestations of TB have no evidence of pulmonary infection on chest radiographs or sputum culture.Cutaneous TB
The incidence of cutaneous TB appears low. In areas such as India or China, where TB prevalence is high, cutaneous manifestations of TB (overt infection or the presence of tuberculids) have been found in less than 0.1% of individuals seen in dermatology clinics.Ocular TB
TB can affect any structure in the eye and typically presents as a granulomatous process. Keratitis, iridocyclitis, intermediate uveitis, retinitis, scleritis, and orbital abscess are within the spectrum of ocular disease. Choroidal tubercles and choroiditis are the most common ocular presentations of TB. Adnexal or orbital disease may be seen with preauricular lymphadenopathy. Because of the wide variability in the disease process, presenting complaints will vary.Most often, patients will complain of blurry vision that may or may not be associated with pain and red eye. In the rare case of orbital disease, proptosis, double vision, or extraocular muscle motility restriction may be the presenting complaint. Preseptal cellulitis in children with spontaneous draining fistula may also occur. In cases of both pulmonary and extrapulmonary TB, there may be ocular findings without ocular complaints.
In patients with confirmed active pulmonary or active nonocular extrapulmonary TB, ocular incidence ranges from 1.4-5.74%. In HIV patients, the incidence of ocular TB may be higher, with a reported prevalence of from 2.8-11.4%.
TB and the legal system
Laws vary from state to state, but communicable-disease laws typically empower public health officials to investigate suspected cases of TB, including potential contacts of persons with TB. In addition, patients may be incarcerated for noncompliance with therapy.Pathophysiology
Infection with M tuberculosis
results most commonly from infected aerosol exposure through the lungs
or mucous membranes. In immunocompetent individuals, this usually
produces a latent/dormant infection; only about 5% of these individuals
later show evidence of clinical disease. (See Etiology.)
Alterations in the host immune system that lead to decreased immune effectiveness can allow M tuberculosis organisms to reactivate, with tubercular disease resulting from a combination of direct effects from the replicating infectious organism and from subsequent inappropriate host immune responses to tubercular antigens.
Molecular typing of M tuberculosis isolates in the United States by restriction fragment-length polymorphism analysis suggests more than one third of new patient occurrences of TB result from person-to-person transmission, with the remainder resulting from reactivation of latent infection.
Verhagen et al demonstrated that large clusters of TB are associated with an increased number of tuberculin skin test-positive contacts, even after adjusting for other risk factors for transmission.[12] The number of positive contacts was significantly lower for index cases with isoniazid-resistant TB compared with index cases with fully-susceptible TB. This suggests that some TB strains may be more transmissible than other strains and that isoniazid resistance is associated with lower transmissibility.
Uveitis caused by TB is the local inflammatory manifestation of a previously acquired primary systemic tubercular infection. There is some debate regarding molecular mimicry, as well as a nonspecific response to noninfectious tubercular antigens, which may produce active ocular inflammation in the absence of bacterial replication.
Alterations in the host immune system that lead to decreased immune effectiveness can allow M tuberculosis organisms to reactivate, with tubercular disease resulting from a combination of direct effects from the replicating infectious organism and from subsequent inappropriate host immune responses to tubercular antigens.
Molecular typing of M tuberculosis isolates in the United States by restriction fragment-length polymorphism analysis suggests more than one third of new patient occurrences of TB result from person-to-person transmission, with the remainder resulting from reactivation of latent infection.
Verhagen et al demonstrated that large clusters of TB are associated with an increased number of tuberculin skin test-positive contacts, even after adjusting for other risk factors for transmission.[12] The number of positive contacts was significantly lower for index cases with isoniazid-resistant TB compared with index cases with fully-susceptible TB. This suggests that some TB strains may be more transmissible than other strains and that isoniazid resistance is associated with lower transmissibility.
Uveitis caused by TB is the local inflammatory manifestation of a previously acquired primary systemic tubercular infection. There is some debate regarding molecular mimicry, as well as a nonspecific response to noninfectious tubercular antigens, which may produce active ocular inflammation in the absence of bacterial replication.
Etiology
M tuberculosis
is a slow-growing, obligate aerobe and a facultative, intracellular
parasite. The organism grows in parallel groups called cords (as seen in
the image below). It retains many stains after decoloration with
acid-alcohol, which is the basis of acid-fast stains.
 Acid-fast bacillus smear showing characteristic cording in Mycobacterium tuberculosis. Mycobacteria, such as M tuberculosis,
are aerobic, non-spore-forming, nonmotile, facultative, intracellular,
curved rods measuring 0.2-0.5 μm by 2-4 μm. Their cell walls contain
mycolic, acid-rich, long-chain glycolipids and phospholipoglycans
(mycocides) that protect mycobacteria from cell lysosomal attack and
also retain red basic fuchsin dye after acid rinsing (acid-fast stain).
Acid-fast bacillus smear showing characteristic cording in Mycobacterium tuberculosis. Mycobacteria, such as M tuberculosis,
are aerobic, non-spore-forming, nonmotile, facultative, intracellular,
curved rods measuring 0.2-0.5 μm by 2-4 μm. Their cell walls contain
mycolic, acid-rich, long-chain glycolipids and phospholipoglycans
(mycocides) that protect mycobacteria from cell lysosomal attack and
also retain red basic fuchsin dye after acid rinsing (acid-fast stain).
Humans are the only known reservoir for M tuberculosis. The organism is spread primarily as an airborne aerosol from infected to noninfected individuals (although transdermal and GI transmission have been reported). These droplets are 1-5 μm in diameter; a single cough can generate 3000 infective droplets, with as few as 10 bacilli needed to initiate infection.
When inhaled, droplet nuclei are deposited within the terminal airspaces of the lung. The organisms grow for 2-12 weeks, until they reach 1000-10,000 in number, which is sufficient to elicit a cellular immune response that can be detected by a reaction to the tuberculin skin test.
Exposure to M tuberculosis can occur when common airspace is shared with an individual who is in the infectious stage of TB.
Mycobacteria are highly antigenic, and they promote a vigorous, nonspecific immune response. Their antigenicity is due to multiple cell wall constituents, including glycoproteins, phospholipids, and wax D, which activate Langerhans cells, lymphocytes, and polymorphonuclear leukocytes.
Because of the ability of M tuberculosis to survive and proliferate within mononuclear phagocytes, which ingest the bacterium, M tuberculosis is able to invade local lymph nodes and spread to extrapulmonary sites, such as the bone marrow, liver, spleen, kidneys, bones, and brain, usually via hematogenous routes.
When a person is infected with M tuberculosis, the infection can take 1 of a variety of paths, most of which do not lead to actual TB. The infection may be cleared by the host immune system or suppressed into an inactive form called latent tuberculosis infection (LTBI), with resistant hosts controlling mycobacterial growth at distant foci before the development of active disease. Patients with LTBI cannot spread disease.
Although mycobacteria are spread by blood throughout the body during initial infection, primary extrapulmonary disease is rare except in immunocompromised hosts. Infants, older persons, or otherwise immunosuppressed hosts are unable to control mycobacterial growth and develop disseminated (primary miliary) TB. Patients who become immunocompromised months to years after primary infection also can develop late, generalized disease.
The lungs are the most common site for the development of TB; 85% of patients with TB present with pulmonary complaints. Extrapulmonary TB can occur as part of a primary or late generalized infection. An extrapulmonary location may also serve as a reactivation site; extrapulmonary reactivation may coexist with pulmonary reactivation.
The most common sites of extrapulmonary disease are mediastinal, retroperitoneal, and cervical (scrofula) lymph nodes; vertebral bodies, adrenals, meninges, and the GI tract. That pathology of these lesions is similar to that in the lungs. (The most common site of tuberculous lymphadenitis (scrofula) is in the neck, along the sternocleidomastoid muscle. It is usually unilateral and causes little or no pain. Advanced cases of tuberculous lymphadenitis may suppurate and form a draining sinus.)
Infected end organs typically have high, regional oxygen tension (as in the kidneys, bones, meninges, eyes, and choroids, and in the apices of the lungs). The principal cause of tissue destruction from M tuberculosis infection is related to the organism's ability to incite intense host immune reactions to antigenic cell wall proteins.
Early tubercles are spherical, 0.5- to 3-mm nodules with 3 or 4 cellular zones demonstrating (1) a central caseation necrosis, (2) an inner cellular zone of epithelioid macrophages and Langhans giant cells admixed with lymphocytes, (3) an outer cellular zone of lymphocytes, plasma cells, and immature macrophages, and (4) a rim of fibrosis (in healing lesions).
Initial lesions may heal and the infection become latent before symptomatic disease occurs. Smaller tubercles may resolve completely. Fibrosis occurs when hydrolytic enzymes dissolve tubercles, and larger lesions are surrounded by a fibrous capsule. Such fibrocaseous nodules usually contain viable mycobacteria and are potential lifelong foci for reactivation or cavitation. Some nodules calcify or ossify and are seen easily on chest radiographs.
Tissues within areas of caseation necrosis have high levels of fatty acids, low pH, and low oxygen tension, all of which inhibit growth of the tubercle bacillus.
If the host is unable to arrest the initial infection, the patient develops progressive, primary TB with tuberculous pneumonia in the lower and middle lobes of the lung. Purulent exudates with large numbers of acid-fast bacilli can be found in sputum and tissue. Subserosal granulomas may rupture into the pleural or pericardial spaces and create serous inflammation and effusions.
With the onset of host-immune response, lesions that develop around mycobacterial foci can be either proliferative or exudative. Both types of lesions develop in the same host, since infective dose and local immunity vary from site to site.
Proliferative lesions develop where the bacillary load is small and host cellular-immune responses dominate. These tubercles are compact, with activated macrophages admixed, and are surrounded by proliferating lymphocytes, plasma cells, and an outer rim of fibrosis. Intracellular killing of mycobacteria is effective, and the bacillary load remains low.
Exudative lesions predominate when large numbers of bacilli are present and host defenses are weak. These loose aggregates of immature macrophages, neutrophils, fibrin, and caseation necrosis are sites of mycobacterial growth. Without treatment, these lesions progress and infection spreads.
Populations at high risk for acquiring the infection also include hospital employees, inner-city residents, nursing home residents, and prisoners.
Increased risk of acquiring active disease occurs with HIV infection, intravenous (IV) drug abuse, alcoholism, diabetes mellitus (3-fold risk), silicosis, immunosuppressive therapy, cancer of the head and neck, hematologic malignancies, end-stage renal disease, intestinal bypass surgery or gastrectomy, chronic malabsorption syndromes, and low body weight. The risk is also higher in infants younger than 5 years.
Tumor necrosis factor-alpha (TNF-a) antagonists, used in the treatment of rheumatoid arthritis, psoriasis, and several other autoimmune disorders, have been associated with a significantly increased risk for TB.[13] Reports have included atypical presentations, extrapulmonary and disseminated disease, and deaths. Patients scheduled to begin therapy with a TNF-α antagonist should be screened for latent TB and counseled regarding the risk of TB.
Immunosuppressive therapy also includes chronic administration of systemic steroids (prednisone or its equivalent, given >15 mg/d for ≥4 wk or more) and/or inhaled steroids. Inhaled steroids, in the absence of systemic steroids, were associated with a relative risk of 1.5.[14]
Smoking has been shown to be a risk factor for TB; smokers who develop TB should be encouraged to stop smoking to decrease the risk of relapse.[15]
Obesity in elderly patients has been associated with a lower risk for pulmonary TB.[16]
Osteoporosis, sclerosis, and bone involvement are more common in children with TB. The epiphyseal bones can be involved due to their high vascularity.
Children do not commonly infect other children, because they rarely develop cough and sputum production is scant. However, cases of child-child and child-adult TB transmission are well-documented. Go to Pediatric Tuberculosis for complete information on this topic.
 Acid-fast bacillus smear showing characteristic cording in Mycobacterium tuberculosis. Mycobacteria, such as M tuberculosis,
are aerobic, non-spore-forming, nonmotile, facultative, intracellular,
curved rods measuring 0.2-0.5 μm by 2-4 μm. Their cell walls contain
mycolic, acid-rich, long-chain glycolipids and phospholipoglycans
(mycocides) that protect mycobacteria from cell lysosomal attack and
also retain red basic fuchsin dye after acid rinsing (acid-fast stain).
Acid-fast bacillus smear showing characteristic cording in Mycobacterium tuberculosis. Mycobacteria, such as M tuberculosis,
are aerobic, non-spore-forming, nonmotile, facultative, intracellular,
curved rods measuring 0.2-0.5 μm by 2-4 μm. Their cell walls contain
mycolic, acid-rich, long-chain glycolipids and phospholipoglycans
(mycocides) that protect mycobacteria from cell lysosomal attack and
also retain red basic fuchsin dye after acid rinsing (acid-fast stain). Humans are the only known reservoir for M tuberculosis. The organism is spread primarily as an airborne aerosol from infected to noninfected individuals (although transdermal and GI transmission have been reported). These droplets are 1-5 μm in diameter; a single cough can generate 3000 infective droplets, with as few as 10 bacilli needed to initiate infection.
When inhaled, droplet nuclei are deposited within the terminal airspaces of the lung. The organisms grow for 2-12 weeks, until they reach 1000-10,000 in number, which is sufficient to elicit a cellular immune response that can be detected by a reaction to the tuberculin skin test.
Exposure to M tuberculosis can occur when common airspace is shared with an individual who is in the infectious stage of TB.
Mycobacteria are highly antigenic, and they promote a vigorous, nonspecific immune response. Their antigenicity is due to multiple cell wall constituents, including glycoproteins, phospholipids, and wax D, which activate Langerhans cells, lymphocytes, and polymorphonuclear leukocytes.
Because of the ability of M tuberculosis to survive and proliferate within mononuclear phagocytes, which ingest the bacterium, M tuberculosis is able to invade local lymph nodes and spread to extrapulmonary sites, such as the bone marrow, liver, spleen, kidneys, bones, and brain, usually via hematogenous routes.
When a person is infected with M tuberculosis, the infection can take 1 of a variety of paths, most of which do not lead to actual TB. The infection may be cleared by the host immune system or suppressed into an inactive form called latent tuberculosis infection (LTBI), with resistant hosts controlling mycobacterial growth at distant foci before the development of active disease. Patients with LTBI cannot spread disease.
Although mycobacteria are spread by blood throughout the body during initial infection, primary extrapulmonary disease is rare except in immunocompromised hosts. Infants, older persons, or otherwise immunosuppressed hosts are unable to control mycobacterial growth and develop disseminated (primary miliary) TB. Patients who become immunocompromised months to years after primary infection also can develop late, generalized disease.
The lungs are the most common site for the development of TB; 85% of patients with TB present with pulmonary complaints. Extrapulmonary TB can occur as part of a primary or late generalized infection. An extrapulmonary location may also serve as a reactivation site; extrapulmonary reactivation may coexist with pulmonary reactivation.
The most common sites of extrapulmonary disease are mediastinal, retroperitoneal, and cervical (scrofula) lymph nodes; vertebral bodies, adrenals, meninges, and the GI tract. That pathology of these lesions is similar to that in the lungs. (The most common site of tuberculous lymphadenitis (scrofula) is in the neck, along the sternocleidomastoid muscle. It is usually unilateral and causes little or no pain. Advanced cases of tuberculous lymphadenitis may suppurate and form a draining sinus.)
Infected end organs typically have high, regional oxygen tension (as in the kidneys, bones, meninges, eyes, and choroids, and in the apices of the lungs). The principal cause of tissue destruction from M tuberculosis infection is related to the organism's ability to incite intense host immune reactions to antigenic cell wall proteins.
Lesions in TB development
The typical TB lesion is epithelioid granuloma with central caseation necrosis. The most common site of the primary lesion is within alveolar macrophages in subpleural regions of the lung. Bacilli proliferate locally and spread through the lymphatics to a hilar node, forming the Ghon complex.Early tubercles are spherical, 0.5- to 3-mm nodules with 3 or 4 cellular zones demonstrating (1) a central caseation necrosis, (2) an inner cellular zone of epithelioid macrophages and Langhans giant cells admixed with lymphocytes, (3) an outer cellular zone of lymphocytes, plasma cells, and immature macrophages, and (4) a rim of fibrosis (in healing lesions).
Initial lesions may heal and the infection become latent before symptomatic disease occurs. Smaller tubercles may resolve completely. Fibrosis occurs when hydrolytic enzymes dissolve tubercles, and larger lesions are surrounded by a fibrous capsule. Such fibrocaseous nodules usually contain viable mycobacteria and are potential lifelong foci for reactivation or cavitation. Some nodules calcify or ossify and are seen easily on chest radiographs.
Tissues within areas of caseation necrosis have high levels of fatty acids, low pH, and low oxygen tension, all of which inhibit growth of the tubercle bacillus.
If the host is unable to arrest the initial infection, the patient develops progressive, primary TB with tuberculous pneumonia in the lower and middle lobes of the lung. Purulent exudates with large numbers of acid-fast bacilli can be found in sputum and tissue. Subserosal granulomas may rupture into the pleural or pericardial spaces and create serous inflammation and effusions.
With the onset of host-immune response, lesions that develop around mycobacterial foci can be either proliferative or exudative. Both types of lesions develop in the same host, since infective dose and local immunity vary from site to site.
Proliferative lesions develop where the bacillary load is small and host cellular-immune responses dominate. These tubercles are compact, with activated macrophages admixed, and are surrounded by proliferating lymphocytes, plasma cells, and an outer rim of fibrosis. Intracellular killing of mycobacteria is effective, and the bacillary load remains low.
Exudative lesions predominate when large numbers of bacilli are present and host defenses are weak. These loose aggregates of immature macrophages, neutrophils, fibrin, and caseation necrosis are sites of mycobacterial growth. Without treatment, these lesions progress and infection spreads.
Risk factors
Four factors contribute to the likelihood of transmission, as follows:- Number of organisms expelled
- Concentration of organisms
- Length of exposure time to contaminated air
- Immune status of the exposed individual
Populations at high risk for acquiring the infection also include hospital employees, inner-city residents, nursing home residents, and prisoners.
Increased risk of acquiring active disease occurs with HIV infection, intravenous (IV) drug abuse, alcoholism, diabetes mellitus (3-fold risk), silicosis, immunosuppressive therapy, cancer of the head and neck, hematologic malignancies, end-stage renal disease, intestinal bypass surgery or gastrectomy, chronic malabsorption syndromes, and low body weight. The risk is also higher in infants younger than 5 years.
Tumor necrosis factor-alpha (TNF-a) antagonists, used in the treatment of rheumatoid arthritis, psoriasis, and several other autoimmune disorders, have been associated with a significantly increased risk for TB.[13] Reports have included atypical presentations, extrapulmonary and disseminated disease, and deaths. Patients scheduled to begin therapy with a TNF-α antagonist should be screened for latent TB and counseled regarding the risk of TB.
Immunosuppressive therapy also includes chronic administration of systemic steroids (prednisone or its equivalent, given >15 mg/d for ≥4 wk or more) and/or inhaled steroids. Inhaled steroids, in the absence of systemic steroids, were associated with a relative risk of 1.5.[14]
Smoking has been shown to be a risk factor for TB; smokers who develop TB should be encouraged to stop smoking to decrease the risk of relapse.[15]
Obesity in elderly patients has been associated with a lower risk for pulmonary TB.[16]
TB in children
In children younger than 5 years, the potential for development of fatal miliary TB or meningeal TB is a significant concern.Osteoporosis, sclerosis, and bone involvement are more common in children with TB. The epiphyseal bones can be involved due to their high vascularity.
Children do not commonly infect other children, because they rarely develop cough and sputum production is scant. However, cases of child-child and child-adult TB transmission are well-documented. Go to Pediatric Tuberculosis for complete information on this topic.
Epidemiology
TB prevalence in the United States
With the improvement of living conditions and the introduction of effective treatment (streptomycin) in the late 1940s, the number of patients in the United States reported to have tuberculosis (TB) underwent a steady decline (126,000 TB patients in 1944; 84,000 in 1953; 22,000 in 1984; 14,000 in 2004), despite explosive growth in the total population (140 million people in 1946, 185 million in 1960, 226 million in 1980).On a national level, the incidence of tuberculosis is at an all-time low. In 2011, a total of 10,521 incident TB cases were reported in the United States, reflecting a 6.4% decline from 2010 to 3.4 cases per 100,000 population.[17]
Demographics of TB in the United States
Nearly half of all TB cases reported (50.4%) have been found to come from 4 states: California, Florida, New York, and Texas.In 2011, more than 60% of cases of TB reportedly occurred among foreign-born persons. Approximately 54% of TB cases involving foreign-born individuals in 2011 were reported in persons from 5 countries: Mexico (21.3%), the Philippines (11.5%), Vietnam (8.2%), India (7.6%), and China (5.6%). An estimated 10-15 million people in the United States have latent TB infection.
International prevalence of TB and M tuberculosis infection
Globally, more than 1 in 3 individuals is infected with tubercle bacillus.An estimated 9.27 million incident TB cases were reported internationally in 2007, an increase from 9.24 million in 2006. However, although the total number of cases increased, the number of cases per capita decreased from a global peak of 142 cases per 100,000 in 2004 to 139 cases per 100,000 in 2007.[1, 18]
Countries with the highest prevalence include Russia, India, Bangladesh, Pakistan, Indonesia, Philippines, Vietnam, Korea, China, Tibet, Hong Kong, Egypt, most sub-Saharan African countries, Brazil, Mexico, Bolivia, Peru, Colombia, Dominican Republic, Ecuador, Puerto Rico, El Salvador, Nicaragua, Haiti, Honduras, and areas undergoing civil war.
The prevalence of TB in countries in Eastern Europe is intermediate. The prevalence of TB is lowest in Costa Rica, western and northern Europe, the United States, Canada, Israel, and most countries in the Caribbean.
Africa, which is home to 13% of the world's population and 13 of the 15 countries with the highest TB incidence, shoulders over 25% of the annual global TB burden in terms of cases and deaths.
Mortality in TB
Internationally, TB a primary infectious cause of morbidity and mortality.As previously noted, WHO estimated that 1.7 million people worldwide died of TB in 2009.[1]
In the United States, 2800 TB deaths are reported annually.
Race prevalence
As previously mentioned, in 2007 almost 60% of cases of TB reportedly occurred among foreign-born persons.This skewed distribution is most likely due to socioeconomic factors. Elevated rates of TB infection are seen in individuals immigrating from Mexico, the Philippines, India, Southeast Asia, Africa, the Caribbean, and Latin America.
Based on 2007 CDC data, the frequency of TB in Hispanics, blacks, and Asians were 7.6, 8.5, and 23.5 times higher than in whites, respectively.[1] However, race is not clearly an independent risk factor, as foreign-born persons account for 77% of TB cases among Hispanics and 96% of TB cases among Asians, but only 29% of TB cases among blacks. Risk is best defined based on social, economic, and medical factors.
Sex prevalence
Despite the fact that TB rates have declined in both sexes in the United States, certain differences exist. TB rates in women decline with age, but in men, rates increase with age. In addition, men are more likely than women to have a positive tuberculin skin test result. The reason for these differences may be social, rather than biologic, in nature.The estimated sex prevalence for TB varies by source, from no sex prevalence to a male-to-female ratio in the United States of 2:1.
Age predilection
Higher rates of TB infection are seen in young, nonwhite adults (peak incidence, 25-40 y) than in white adults. In addition, white adults manifest the disease later (peak incidence, age 70 y) than do nonwhite persons.In the United States, more than 60% of TB cases occur in persons aged 25-64 years; however, the age-specific risk is highest in persons older than age 65 years.[1]
TB is uncommon in children aged 5-15 years.
Prognosis
Among published studies involving DOT treatment of tuberculosis (TB), the rate of recurrence ranges from 0-14%.[19] In countries with low TB rates, recurrences usually occur within 12 months of treatment completion and are due to relapse.[20] In
countries with higher TB rates, most recurrences after appropriate
treatment are probably due to reinfection rather than relapse.[21]
Full resolution is generally expected with few complications in cases of non-MDR-TB and non-XDR-TB, when the drug regimen is completed.
Poor prognostic markers include extrapulmonary involvement, an immunocompromised state, older age, and a history of previous treatment.
In a prospective study of 199 patients with TB in Malawi, 12 (6%) died. Risk factors for dying were reduced baseline TNF alpha response to stimulation (with heat-killed M tuberculosis), low body mass index, and elevated respiratory rate at TB diagnosis.[22]
Full resolution is generally expected with few complications in cases of non-MDR-TB and non-XDR-TB, when the drug regimen is completed.
Poor prognostic markers include extrapulmonary involvement, an immunocompromised state, older age, and a history of previous treatment.
In a prospective study of 199 patients with TB in Malawi, 12 (6%) died. Risk factors for dying were reduced baseline TNF alpha response to stimulation (with heat-killed M tuberculosis), low body mass index, and elevated respiratory rate at TB diagnosis.[22]
Patient Education
For patient education information, see the Bacterial and Viral Infections Center, as well as Tuberculosis.
Additional information can be found through the following sources:
Along with the differentials listed in the next section, conditions to consider in the diagnosis of patients with symptoms of tuberculosis (TB) include the following:
Specific dermatologic considerations in the identification of TB
Differentiate primary-inoculation TB from ulceroglandular complexes and mycobacterioses.
Differentiate TB verrucosa cutis from diseases such as North American blastomycosis, chromoblastomycosis, iododerma and bromoderma, chronic vegetative pyoderma, verruca vulgaris, verrucous carcinoma, verrucous atypical mycobacterial infection, and verrucous lupus vulgaris.
Differentiate miliary TB of the skin (which appears as small, noncharacteristic, erythematous, papular or purpuric lesions) from drug reactions)
Differentiate scrofuloderma from supportive lymphadenitis with sinus-tract formation, such as blastomycosis and coccidioidomycosis.
Differentiate TB cutis orificialis from glossitis, apotheosis, and deep fungal infections.
Differentiate lupus vulgaris from lupoid rosacea, deep fungal or atypical mycobacterial infection, chronic granulomatous disease, granulomatous rosacea, and Wegener granulomatosis.
Differentiate erythema induratum from nodular panniculitides (eg, Weber-Christian disease) and nodular vasculitides (eg, syphilitic gumma, nodular pernio).
Differentiate papulonecrotic tuberculid from other papulonecrotic entities, such as leukocytoclastic vasculitis, lymphomatoid papulosis, papular eczema, and prurigo simplex with neurotic excoriation.
Differentiate lichen scrofulosorum from keratosis spinulosa, lichenoid sarcoid, and lichenoid secondary syphilis.
If vertebral involvement (Pott disease) or brain involvement is suspected in a patient, it is important to consider that a delay in treatment could have severe repercussions for the patient (compression of the spinal cord and/or paraplegia); further evaluation is necessary with computed tomography (CT) scanning or magnetic resonance imaging (MRI).
Additional information can be found through the following sources:
- World Health Organization Tuberculosis
Clinical Presentation
History
Overview
The following factors increase the likelihood that a patient will have tuberculosis (TB):
- HIV infection
- History of a positive purified protein derivative (PPD) test result
- History of prior TB treatment
- TB exposure
- Travel to or emigration from a TB endemic area
- Homelessness, shelter-dwelling, incarceration
- Cough
- Weight loss/anorexia
- Fever
- Night sweats
- Hemoptysis
- Chest pain
Genitourinary symptoms are less common in patients with TB. In women, dysuria, hematuria, and frequent urination may be present. In men, painful scrotal mass, prostatitis, orchitis, and epididymitis may be present.
Signs and symptoms of extrapulmonary TB may be nonspecific. They can include leukocytosis, anemia, and hyponatremia due to the release of ADH (antidiuretic hormone)-like hormone from affected lung tissue.
Elderly individuals with TB may not display typical signs and symptoms of TB infection because they may not mount a good immune response. Active TB infection in this age group may manifest as nonresolving pneumonitis.
Pulmonary tuberculosis (TB)
Typical symptoms of pulmonary TB include a productive cough, fever, and weight loss. Patients with pulmonary TB occasionally present with hemoptysis or chest pain. Other systemic symptoms include anorexia, fatigue, and night sweats.
Tuberculous meningitis
Patients with tuberculous meningitis may present with a headache that is either intermittent or persistent for 2-3 weeks. Subtle mental status changes may progress to coma over a period of days to weeks. Fever may be low-grade or absent.
Go to Tuberculous Meningitis for complete information on this topic.
Skeletal TB
The most common site of skeletal TB involvement is the spine (Pott disease). Symptoms include back pain or stiffness. Lower-extremity paralysis occurs in up to half of patients with undiagnosed Pott disease. Tuberculous arthritis usually involves only 1 joint. Although any joint may be involved, the hips and knees are affected most commonly, followed by the ankle, elbow, wrist, and shoulder. Pain may precede radiographic changes by weeks to months.
Genitourinary TB
Reported symptoms of genitourinary TB include flank pain, dysuria, and frequency. In men, genital TB may manifest as epididymitis or a scrotal mass. In women, genital TB may mimic pelvic inflammatory disease. TB is the cause of approximately 10% of sterility cases in women worldwide and of approximately 1% in industrialized countries.
Go to Tuberculosis of the Genitourinary System and Imaging of Genitourinary Tuberculosis for complete information on these topics.
Gastrointestinal TB
Any site along the gastrointestinal tract may become infected. Symptoms of gastrointestinal TB are referable to the site infected, including the following: nonhealing ulcers of the mouth or anus; difficulty swallowing (with esophageal disease); abdominal pain mimicking peptic ulcer disease (with stomach or duodenal infection); malabsorption (with infection of the small intestine); and pain, diarrhea, or hematochezia (with infection of the colon).Physical Examination
Physical examination findings associated with TB depend on the organs involved.
Patients with pulmonary TB have abnormal breath sounds, especially over the upper lobes or involved areas. Rales or bronchial breath signs may be noted, indicating lung consolidation.
Signs of extrapulmonary TB differ according to the tissues involved. Signs may include confusion, coma, neurologic deficit, chorioretinitis, lymphadenopathy, and cutaneous lesions.
Lymphadenopathy in TB takes occurs as painless swelling of 1 or more lymph nodes, usually bilaterally; typically, anterior or posterior cervical chain or supraclavicular may be present.
The absence of any significant physical findings does not exclude active TB. In high-risk patients, classic symptoms are often absent, particularly in patients who are immunocompromised or elderly. Up to 20% of patients with active TB may deny symptoms. Therefore, sputum sampling is essential when chest radiography findings are consistent with TB
Differential Diagnoses
Diagnostic Considerations
Conditions to considerAlong with the differentials listed in the next section, conditions to consider in the diagnosis of patients with symptoms of tuberculosis (TB) include the following:
- Blastomycosis
- Tularemia
- Actinomycosis
- Hidradenitis suppurativa
- Eosinophilic granuloma
- Mycobacterium avium-intracellulare infection
- Mycobacterium chelonae infection
- Mycobacterium fortuitum infection
- Mycobacterium gordonae infection
- Mycobacterium kansasii infection
- Mycobacterium marinum infection
- Mycobacterium xenopi infection
- Endemic syphilis
- Erythema induratum (nodular vasculitis)
- Erythema nodosum
- Leishmaniasis
- Leprosy
- Catscratch disease
- Dermatitis herpetiformis
- Discoid lupus erythematosus
- Pustular psoriasis
- Squamous cell carcinoma
- Syphilis
- Syringoma
- Orbital cellulitis
- Preseptal cellulitis
- Rheumatoid arthritis
Specific dermatologic considerations in the identification of TB
Differentiate primary-inoculation TB from ulceroglandular complexes and mycobacterioses.
Differentiate TB verrucosa cutis from diseases such as North American blastomycosis, chromoblastomycosis, iododerma and bromoderma, chronic vegetative pyoderma, verruca vulgaris, verrucous carcinoma, verrucous atypical mycobacterial infection, and verrucous lupus vulgaris.
Differentiate miliary TB of the skin (which appears as small, noncharacteristic, erythematous, papular or purpuric lesions) from drug reactions)
Differentiate scrofuloderma from supportive lymphadenitis with sinus-tract formation, such as blastomycosis and coccidioidomycosis.
Differentiate TB cutis orificialis from glossitis, apotheosis, and deep fungal infections.
Differentiate lupus vulgaris from lupoid rosacea, deep fungal or atypical mycobacterial infection, chronic granulomatous disease, granulomatous rosacea, and Wegener granulomatosis.
Differentiate erythema induratum from nodular panniculitides (eg, Weber-Christian disease) and nodular vasculitides (eg, syphilitic gumma, nodular pernio).
Differentiate papulonecrotic tuberculid from other papulonecrotic entities, such as leukocytoclastic vasculitis, lymphomatoid papulosis, papular eczema, and prurigo simplex with neurotic excoriation.
Differentiate lichen scrofulosorum from keratosis spinulosa, lichenoid sarcoid, and lichenoid secondary syphilis.
Differential Diagnoses
- Actinomycosis
- Aspergillosis
- Bronchiectasis
- Histoplasmosis
- Lung Abscess
- Lung Cancer, Non-Small Cell
- Nocardiosis
- Pericarditis, Constrictive
- Pneumonia, Fungal
- Pott Disease (Tuberculous Spondylitis)
Workup
Approach Considerations
Obtain the following laboratory tests:
If chest radiography findings suggest TB and sputum smear is positive for acid-fast bacilli, initiate treatment for TB.
Ziehl-Neelsen staining of sputum is a simple 5-step process that takes approximately 10 minutes to accomplish. While highly specific for mycobacteria, this stain is relatively insensitive, and detection requires at least 10,000 bacilli per mL; most clinical laboratories currently use a more sensitive auramine-rhodamine fluorescent stain (auramine O).
Routine culture uses a nonselective egg medium (Lowenstein-Jensen or Middlebrook 7H10) and often requires more than 3-4 weeks to grow because of the 22-hour doubling time of M tuberculosis. Radiometric broth culture (BACTEC radiometric system) of clinical specimens significantly reduced the time (10-14 d) for mycobacterial recovery. Newer broth culture media and systems for isolation are available for use in clinical laboratories based on a fluorescent rather than a radioactive indicator. The indicator is inhibited by oxygen; as mycobacteria metabolize substrates in the tubes and use the oxygen, the tube begins to fluoresce.[23]
Deoxyribonucleic acid (DNA) probes specific for mycobacterial ribosomal ribonucleic acid (RNA) identify species of clinically significant isolates after recovery. In tissue, polymerase chain reaction (PCR) amplification techniques can be used to detect M tuberculosis -specific DNA sequences and thus, small numbers of mycobacteria in clinical specimens.[24, 25]
Extrapulmonary involvement occurs in one fifth of all TB cases, although 60% of patients with extrapulmonary manifestations of TB have no evidence of pulmonary infection on chest radiograph or sputum culture. Ocular TB can be especially difficult to identify, owing to its mimicry and its lack of accessible sampling; a high index of suspicion is required.
The hallmark of extrapulmonary TB histopathology is the caseating granuloma, consisting of giant cells with central caseating necrosis. Rarely, if ever, are any TB bacilli seen.
Altered mental status, neck stiffness, decreased level of consciousness, increased intracranial pressure, and cranial nerve involvement can indicate tuberculosis meningitis or tuberculoma. TB can directly seed the meninges and, if suspected, performing a lumbar puncture for evaluation of the cerebrospinal fluid is necessary. In addition, a tuberculoma can be substantiated based on an increase in intracranial pressure and computed tomography (CT) scanning/magnetic resonance imaging (MRI).
If vertebral involvement (Pott disease) or brain involvement is suspected, it is important to consider that a delay in treatment could have severe repercussions for the patient (compression of the spinal cord and/or paraplegia); further evaluation is necessary with CT scanning or MRI.
Chest radiographs in children with TB may show only hilar lymphadenopathy or a patchy infiltrate. Most children with TB can be treated with isoniazid and rifampin for 6 months, along with pyrazinamide for the first 2 months if the culture from the source case is fully susceptible. Gastric aspirates or biopsies are not necessary if positive cultures have been obtained from the source case.
Go to Pediatric Tuberculosis for complete information on this topic.
In a study from Durban, South Africa, nearly 20% of patients starting ART had undiagnosed, culture-positive pulmonary TB. Neither cough nor acid-fast bacillus smear were sufficiently sensitive for screening. TB sputum cultures should be attempted before ART initiation in areas with a high prevalence of TB.[29]
Patients with TB must be tested for HIV, and patients with HIV need periodic evaluation for TB with tuberculin skin testing and/or chest radiography. Patients with HIV and a positive tuberculin skin test result develop active TB at a rate of 3-16% per year.
Patients with TB and HIV are more likely to have disseminated disease and less likely to have upper-lobe infiltrates or classic cavitary pulmonary disease. Patients with a CD4 count of less than 200/μL may have mediastinal adenopathy with infiltrates.
- Sputum for acid fast smear and culture
- Complete blood count (CBC)
- Chemistries, including alanine aminotransferase (ALT) or aspartate aminotransferase (AST)
- Alkaline phosphatase
- Total bilirubin
- Uric acid
- Creatinine
- HIV serology in all patients with tuberculosis (TB) and unknown HIV status
If chest radiography findings suggest TB and sputum smear is positive for acid-fast bacilli, initiate treatment for TB.
Ziehl-Neelsen staining of sputum is a simple 5-step process that takes approximately 10 minutes to accomplish. While highly specific for mycobacteria, this stain is relatively insensitive, and detection requires at least 10,000 bacilli per mL; most clinical laboratories currently use a more sensitive auramine-rhodamine fluorescent stain (auramine O).
Routine culture uses a nonselective egg medium (Lowenstein-Jensen or Middlebrook 7H10) and often requires more than 3-4 weeks to grow because of the 22-hour doubling time of M tuberculosis. Radiometric broth culture (BACTEC radiometric system) of clinical specimens significantly reduced the time (10-14 d) for mycobacterial recovery. Newer broth culture media and systems for isolation are available for use in clinical laboratories based on a fluorescent rather than a radioactive indicator. The indicator is inhibited by oxygen; as mycobacteria metabolize substrates in the tubes and use the oxygen, the tube begins to fluoresce.[23]
Deoxyribonucleic acid (DNA) probes specific for mycobacterial ribosomal ribonucleic acid (RNA) identify species of clinically significant isolates after recovery. In tissue, polymerase chain reaction (PCR) amplification techniques can be used to detect M tuberculosis -specific DNA sequences and thus, small numbers of mycobacteria in clinical specimens.[24, 25]
Extrapulmonary involvement occurs in one fifth of all TB cases, although 60% of patients with extrapulmonary manifestations of TB have no evidence of pulmonary infection on chest radiograph or sputum culture. Ocular TB can be especially difficult to identify, owing to its mimicry and its lack of accessible sampling; a high index of suspicion is required.
The hallmark of extrapulmonary TB histopathology is the caseating granuloma, consisting of giant cells with central caseating necrosis. Rarely, if ever, are any TB bacilli seen.
Altered mental status, neck stiffness, decreased level of consciousness, increased intracranial pressure, and cranial nerve involvement can indicate tuberculosis meningitis or tuberculoma. TB can directly seed the meninges and, if suspected, performing a lumbar puncture for evaluation of the cerebrospinal fluid is necessary. In addition, a tuberculoma can be substantiated based on an increase in intracranial pressure and computed tomography (CT) scanning/magnetic resonance imaging (MRI).
If vertebral involvement (Pott disease) or brain involvement is suspected, it is important to consider that a delay in treatment could have severe repercussions for the patient (compression of the spinal cord and/or paraplegia); further evaluation is necessary with CT scanning or MRI.
Tuberculin sensitivity
Tuberculin sensitivity develops 2-10 weeks after infection and usually is lifelong.Multidrug-resistant TB
Symptoms and radiographic findings do not differentiate MDR-TB from fully susceptible TB. Suspect MDR-TB if the patient has a history of previous treatment for TB, was born in or lived in a country with a high prevalence of MDR-TB, has a known exposure to a MDR-TB case, or is clinically progressing despite standard TB therapy. Susceptibilities should be repeated if cultures remain positive after 2 months, even when initial susceptibilities did not reveal any resistance.Pregnancy
Pregnancy provides an opportunity to screen for TB; all pregnant women can undergo tuberculin skin testing. If skin-testing results are positive, chest radiography can be performed with lead shielding. Chest radiography should not be delayed during the first 3 months of pregnancy in patients with suggestive symptoms.TB in children
Postnatal TB is contracted via the airborne route. The most common findings of postnatal TB include adenopathy and a lung infiltrate. However, the chest radiographic findings may be normal in infants with disseminated disease.Chest radiographs in children with TB may show only hilar lymphadenopathy or a patchy infiltrate. Most children with TB can be treated with isoniazid and rifampin for 6 months, along with pyrazinamide for the first 2 months if the culture from the source case is fully susceptible. Gastric aspirates or biopsies are not necessary if positive cultures have been obtained from the source case.
Go to Pediatric Tuberculosis for complete information on this topic.
Human immunodeficiency virus
Individuals infected with HIV are at increased risk for TB, beginning within the first year of HIV infection.[26] Based on historical data, the initiation of antiretroviral therapy (ART) decreases the risk of developing TB in these patients.[27] Risk for TB remains higher the first 3 months after starting ART; risk was highest among patients with a baseline CD4 count of less than 200/μL, higher baseline HIV-1 RNA level (relative hazard 1.93 for every log increase in baseline HIV-1 RNA), a history of injection drug use, and male sex.[28]In a study from Durban, South Africa, nearly 20% of patients starting ART had undiagnosed, culture-positive pulmonary TB. Neither cough nor acid-fast bacillus smear were sufficiently sensitive for screening. TB sputum cultures should be attempted before ART initiation in areas with a high prevalence of TB.[29]
Patients with TB must be tested for HIV, and patients with HIV need periodic evaluation for TB with tuberculin skin testing and/or chest radiography. Patients with HIV and a positive tuberculin skin test result develop active TB at a rate of 3-16% per year.
Patients with TB and HIV are more likely to have disseminated disease and less likely to have upper-lobe infiltrates or classic cavitary pulmonary disease. Patients with a CD4 count of less than 200/μL may have mediastinal adenopathy with infiltrates.
Cultures and Alternative Methodologies
Patients
suspected of having tuberculosis (TB) should submit sputum for smear
and culture. Sputum should be collected in the early morning on 3
consecutive days. In hospitalized patients, sputum may be collected
every 8 hours. However, the absence of a positive smear result does not
exclude active TB infection.
Approximately 35% of culture-positive specimens are associated with a negative smear result.
In patients without spontaneous sputum production, sputum induction with hypertonic saline should be attempted.[30] Early-morning gastric aspirate may also produce a good specimen, especially in children.
Patients diagnosed with active TB should undergo sputum analysis for M tuberculosis weekly until sputum conversion is documented. Monitoring for toxicity includes baseline and periodic liver enzymes, CBC count, and serum creatinine.
Another option is fiberoptic bronchoscopy with transbronchial biopsy and bronchial washings. Biopsy of bone marrow, liver, or blood cultures is occasionally necessary and may be helpful.
Traditional mycobacterial cultures require weeks for growth and identification. Newer technologies, including ribosomal RNA probes and DNA PCR, allow identification within 24 hours. The DNA probes are approved for direct testing on smear-positive or smear-negative sputa. However, smear-positive specimens yielded higher sensitivity.
Culture for acid-fast bacilli (AFB) is the most specific and allows direct identification and susceptibility of the causative organism; however, access to the organisms may require lymph node/sputum analysis, bronchoalveolar lavage, or aspirate of cavity fluid or bone marrow. Unfortunately, obtaining the test results is slow (3-8 wk), and they have a very low positivity in some forms of disease.
AFB stain is quick but requires a very high organism load for positivity. This is more useful in patients with pulmonary disease, but a delay in diagnosis can increase mortality, as other diagnostic testing may need to be considered.
Blood cultures using mycobacteria-specific, radioisotope-labeled systems help to establish the diagnosis of active TB. Mycobacterial bacteremia (bacillemia) is detectable using blood cultures only if specialized systems are used. The bacilli have specific nutrient growth requirements not met by routine culture systems.
Such blood cultures should be used for all patients with HIV who are suspected of having TB, because bacillemia is particularly prevalent in this population. If available, such cultures should be used for any patient highly suspected of having active TB. One study found an incidence of 88% mycobacterial infection (66% TB, 22% Mycobacterium avium complex [MAC]) detected by blood culture in stage IV HIV disease).
Approximately 35% of culture-positive specimens are associated with a negative smear result.
In patients without spontaneous sputum production, sputum induction with hypertonic saline should be attempted.[30] Early-morning gastric aspirate may also produce a good specimen, especially in children.
Patients diagnosed with active TB should undergo sputum analysis for M tuberculosis weekly until sputum conversion is documented. Monitoring for toxicity includes baseline and periodic liver enzymes, CBC count, and serum creatinine.
Another option is fiberoptic bronchoscopy with transbronchial biopsy and bronchial washings. Biopsy of bone marrow, liver, or blood cultures is occasionally necessary and may be helpful.
Traditional mycobacterial cultures require weeks for growth and identification. Newer technologies, including ribosomal RNA probes and DNA PCR, allow identification within 24 hours. The DNA probes are approved for direct testing on smear-positive or smear-negative sputa. However, smear-positive specimens yielded higher sensitivity.
Culture for acid-fast bacilli (AFB) is the most specific and allows direct identification and susceptibility of the causative organism; however, access to the organisms may require lymph node/sputum analysis, bronchoalveolar lavage, or aspirate of cavity fluid or bone marrow. Unfortunately, obtaining the test results is slow (3-8 wk), and they have a very low positivity in some forms of disease.
AFB stain is quick but requires a very high organism load for positivity. This is more useful in patients with pulmonary disease, but a delay in diagnosis can increase mortality, as other diagnostic testing may need to be considered.
Blood cultures using mycobacteria-specific, radioisotope-labeled systems help to establish the diagnosis of active TB. Mycobacterial bacteremia (bacillemia) is detectable using blood cultures only if specialized systems are used. The bacilli have specific nutrient growth requirements not met by routine culture systems.
Such blood cultures should be used for all patients with HIV who are suspected of having TB, because bacillemia is particularly prevalent in this population. If available, such cultures should be used for any patient highly suspected of having active TB. One study found an incidence of 88% mycobacterial infection (66% TB, 22% Mycobacterium avium complex [MAC]) detected by blood culture in stage IV HIV disease).
Drug Susceptibility Testing
Because conventional drug susceptibility tests for drug-resistant M tuberculosis
take at least 3-8 weeks, Choi et al recommend direct DNA sequencing
analysis as a rapid and useful method for detecting drug-resistant TB.
In their clinical study of the use of direct DNA sequencing analysis for
detecting drug-resistant TB, turnaround time of the direct DNA
sequencing analysis was 3.8 +/- 1.8 days.
A total of 113 sputum specimens from 111 patients in the study were tested for genes conferring resistance to isoniazid, rifampin, ethambutol, and pyrazinamide, and the results were compared with drug susceptibility tests. The sensitivity and specificity of the assay were 63.6% and 94.6% for isoniazid, 96.2 and 93.9% for rifampin, 69.2% and 97.5% for ethambutol, and 100% and 92.6% for pyrazinamide, respectively.[31]
An automated molecular test that uses sputum samples for the detection of M tuberculosis and resistance to rifampin has been developed. In studies conducted in low-income countries, the sensitivity for TB was 98.3% (CI, 97-99%) using a single smear-positive sputum sample and 76.9% (CI, 72.4-80.8%) using a single smear-negative sputum sample. Sensitivity from smear-negative sputum samples increased to 90.2% when 3 samples were tested. The test correctly identified 94.4% (CI, 90.8-96.6%) of rifampin-resistant organisms and 98.3% (CI, 97.1-99%) of rifampin-sensitive organisms.[32, 33]
Microscopic-observation drug susceptibility (MODS) and thin-layer agar (TLA) assays are inexpensive, rapid alternatives to conventional methods or molecular methods for TB drug susceptibility testing. WHO endorsed the MODS assay, as a direct or indirect test, for rapid screening of patients with suspected MDR-TB. The evidence is insufficient to recommend the use of the TLA assay for rapid screening, but this assay is a promising diagnostic technique. Further research is encouraged.[34]
A total of 113 sputum specimens from 111 patients in the study were tested for genes conferring resistance to isoniazid, rifampin, ethambutol, and pyrazinamide, and the results were compared with drug susceptibility tests. The sensitivity and specificity of the assay were 63.6% and 94.6% for isoniazid, 96.2 and 93.9% for rifampin, 69.2% and 97.5% for ethambutol, and 100% and 92.6% for pyrazinamide, respectively.[31]
An automated molecular test that uses sputum samples for the detection of M tuberculosis and resistance to rifampin has been developed. In studies conducted in low-income countries, the sensitivity for TB was 98.3% (CI, 97-99%) using a single smear-positive sputum sample and 76.9% (CI, 72.4-80.8%) using a single smear-negative sputum sample. Sensitivity from smear-negative sputum samples increased to 90.2% when 3 samples were tested. The test correctly identified 94.4% (CI, 90.8-96.6%) of rifampin-resistant organisms and 98.3% (CI, 97.1-99%) of rifampin-sensitive organisms.[32, 33]
Microscopic-observation drug susceptibility (MODS) and thin-layer agar (TLA) assays are inexpensive, rapid alternatives to conventional methods or molecular methods for TB drug susceptibility testing. WHO endorsed the MODS assay, as a direct or indirect test, for rapid screening of patients with suspected MDR-TB. The evidence is insufficient to recommend the use of the TLA assay for rapid screening, but this assay is a promising diagnostic technique. Further research is encouraged.[34]
Chest Radiography
Obtain
a chest radiograph to evaluate for possible associated pulmonary
findings (demonstrated in the images below). A traditional lateral and
PA view should be ordered. In addition, an apical lordotic view may
permit better visualization of the apices and increase the sensitivity
of chest radiography for indolent or dormant disease.
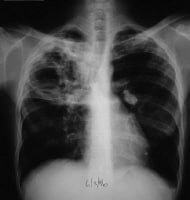 This radiograph shows a patient with typical radiographic findings of tuberculosis.
This radiograph shows a patient with typical radiographic findings of tuberculosis. 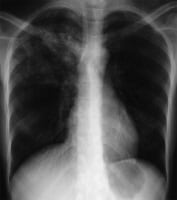 Anteroposterior
chest radiograph in a young ED patient presenting with cough and
malaise. The radiograph shows a classic posterior segment right upper
lobe density consistent with active tuberculosis. This woman was
admitted to isolation and started empirically on a 4-drug regimen in the
ED. Tuberculosis was confirmed on sputum testing. Image courtesy of
Remote Medicine, remotemedicine.org.
Anteroposterior
chest radiograph in a young ED patient presenting with cough and
malaise. The radiograph shows a classic posterior segment right upper
lobe density consistent with active tuberculosis. This woman was
admitted to isolation and started empirically on a 4-drug regimen in the
ED. Tuberculosis was confirmed on sputum testing. Image courtesy of
Remote Medicine, remotemedicine.org. 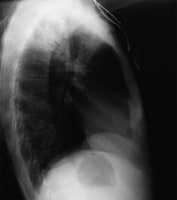 Lateral
chest radiograph of a patient with posterior segment right upper lobe
density consistent with active tuberculosis. Image courtesy of Remote
Medicine, remotemedicine.org. The chest film is also useful to
screen for sarcoidosis, which closely imitates the clinical course of
ocular TB. Radiologists look more decisively for signs of TB or sarcoid
if the requesting physician simply asks to rule out sarcoid or TB.
Lateral
chest radiograph of a patient with posterior segment right upper lobe
density consistent with active tuberculosis. Image courtesy of Remote
Medicine, remotemedicine.org. The chest film is also useful to
screen for sarcoidosis, which closely imitates the clinical course of
ocular TB. Radiologists look more decisively for signs of TB or sarcoid
if the requesting physician simply asks to rule out sarcoid or TB.
Chest radiographs may show a patchy or nodular infiltrate (as seen in the image below). TB may be found in any part of the lung, but upper-lobe involvement is most common. The lordotic view may better demonstrate apical abnormalities.
Primary TB is more likely to mimic the appearance of routine community-acquired pneumonia on chest radiography, in contrast to reactivation TB. Studies have shown that either may be associated with pleural effusion or cavitation.
Various patterns may be seen, as follows (these are further discussed below):
In primary active TB, radiographic features of pulmonary tuberculosis are nonspecific, sometimes even normal. The chest radiograph typically shows a pneumonialike picture of an infiltrative process in the middle or lower lung regions, often associated with hilar adenopathy and/or atelectasis.
In classic reactivation TB, pulmonary lesions are located in the posterior segment of the right upper lobe, apicoposterior segment of the left upper lobe, and apical segments of the lower lobes. Cavitation is most common; healing of tubercular regions results in the development of a scar with loss of lung parenchymal volume and calcification.
In the presence of HIV or another immunosuppressive disease, lesions are often atypical. Up to 20% of patients who are HIV positive with active disease have normal chest radiographic findings.
Old, healed TB presents differently, with dense pulmonary nodules found, with or without calcifications, in the hilar or upper lobes. Smaller nodules, with or without fibrotic scars, can be seen in the upper lobes. Nodules and fibrotic lesions are well demarcated, have sharp margins, and are dense. Persons with nodular or fibrotic scars with positive chest radiographic findings and positive PPD results should be treated as latent carriers. Calcified nodular lesions (granulomas) or apical pleural thickening has a lower risk of conversion.
In disseminated/miliary tuberculosis, the chest radiograph commonly shows a miliary pattern, with 2-mm nodules that are histologically granulomas disseminated like millet seeds throughout the lung; however, chest radiographic patterns can vary and can include upper lobe infiltrates with or without cavitation.
In pleural tuberculosis, the pleural space can be involved in 2 ways: a hypersensitivity response with pleuritic pain and fever, or an empyema that can be seen on chest radiograph with associated pleural effusions.
See the following articles for more information:
 This radiograph shows a patient with typical radiographic findings of tuberculosis.
This radiograph shows a patient with typical radiographic findings of tuberculosis.  Anteroposterior
chest radiograph in a young ED patient presenting with cough and
malaise. The radiograph shows a classic posterior segment right upper
lobe density consistent with active tuberculosis. This woman was
admitted to isolation and started empirically on a 4-drug regimen in the
ED. Tuberculosis was confirmed on sputum testing. Image courtesy of
Remote Medicine, remotemedicine.org.
Anteroposterior
chest radiograph in a young ED patient presenting with cough and
malaise. The radiograph shows a classic posterior segment right upper
lobe density consistent with active tuberculosis. This woman was
admitted to isolation and started empirically on a 4-drug regimen in the
ED. Tuberculosis was confirmed on sputum testing. Image courtesy of
Remote Medicine, remotemedicine.org.  Lateral
chest radiograph of a patient with posterior segment right upper lobe
density consistent with active tuberculosis. Image courtesy of Remote
Medicine, remotemedicine.org. The chest film is also useful to
screen for sarcoidosis, which closely imitates the clinical course of
ocular TB. Radiologists look more decisively for signs of TB or sarcoid
if the requesting physician simply asks to rule out sarcoid or TB.
Lateral
chest radiograph of a patient with posterior segment right upper lobe
density consistent with active tuberculosis. Image courtesy of Remote
Medicine, remotemedicine.org. The chest film is also useful to
screen for sarcoidosis, which closely imitates the clinical course of
ocular TB. Radiologists look more decisively for signs of TB or sarcoid
if the requesting physician simply asks to rule out sarcoid or TB. Chest radiographs may show a patchy or nodular infiltrate (as seen in the image below). TB may be found in any part of the lung, but upper-lobe involvement is most common. The lordotic view may better demonstrate apical abnormalities.
Primary TB is more likely to mimic the appearance of routine community-acquired pneumonia on chest radiography, in contrast to reactivation TB. Studies have shown that either may be associated with pleural effusion or cavitation.
Various patterns may be seen, as follows (these are further discussed below):
- Cavity formation - Indicates advanced infection and is associated with a high bacterial load
- Noncalcified round infiltrates - May be confused with lung carcinoma
- Homogeneously calcified nodules (usually 5-20 mm) - Tuberculomas; represent old infection rather than active disease
- Miliary TB - Characterized by the appearance of numerous small, nodular lesions that resemble millet seeds on chest radiography (Go to Miliary Tuberculosis for complete information on this topic.
In primary active TB, radiographic features of pulmonary tuberculosis are nonspecific, sometimes even normal. The chest radiograph typically shows a pneumonialike picture of an infiltrative process in the middle or lower lung regions, often associated with hilar adenopathy and/or atelectasis.
In classic reactivation TB, pulmonary lesions are located in the posterior segment of the right upper lobe, apicoposterior segment of the left upper lobe, and apical segments of the lower lobes. Cavitation is most common; healing of tubercular regions results in the development of a scar with loss of lung parenchymal volume and calcification.
In the presence of HIV or another immunosuppressive disease, lesions are often atypical. Up to 20% of patients who are HIV positive with active disease have normal chest radiographic findings.
Old, healed TB presents differently, with dense pulmonary nodules found, with or without calcifications, in the hilar or upper lobes. Smaller nodules, with or without fibrotic scars, can be seen in the upper lobes. Nodules and fibrotic lesions are well demarcated, have sharp margins, and are dense. Persons with nodular or fibrotic scars with positive chest radiographic findings and positive PPD results should be treated as latent carriers. Calcified nodular lesions (granulomas) or apical pleural thickening has a lower risk of conversion.
In disseminated/miliary tuberculosis, the chest radiograph commonly shows a miliary pattern, with 2-mm nodules that are histologically granulomas disseminated like millet seeds throughout the lung; however, chest radiographic patterns can vary and can include upper lobe infiltrates with or without cavitation.
In pleural tuberculosis, the pleural space can be involved in 2 ways: a hypersensitivity response with pleuritic pain and fever, or an empyema that can be seen on chest radiograph with associated pleural effusions.
See the following articles for more information:
CT Scanning and Technetium Scanning
CT scanning
CT scanning of the chest may help to better define abnormalities in patients with vague findings on chest radiography.If vertebral involvement (Pott disease) or brain involvement is suspected in a patient, it is important to consider that a delay in treatment could have severe repercussions for the patient (compression of the spinal cord and/or paraplegia); further evaluation is necessary with computed tomography (CT) scanning or magnetic resonance imaging (MRI).
Technetium scanning
Technetium-99m (99m Tc) methoxy isobutyl isonitrile single-photon emission CT (SPECT) scanning for solitary pulmonary nodules yields a high predictive value for distinguishing TB from malignancy. Therefore, it has the potential to serve as a low-cost alternative when positron emission tomography (PET) scanning is not available, especially in endemic areas.[35]Tuberculin Skin Testing and IGRA
The primary screening for TB infection (active or latent) is the tuberculin skin test with purified protein derivative (PPD).
The mechanism of tuberculin skin testing is based on the fact that latent TB infection induces a strong cell-mediated immune response by measuring the delayed-type hypersensitivity response to intradermal inoculation of tuberculin PPD.
The PPD test is given in an intradermal injection of 5 units of purified protein derivative, preferably with a 26-, 27-, or 30-gauge needle. These delayed-type hypersensitivity tests should be read between 48 and 72 hours after administration.
A negative response in immunologically intact individuals measures less than 5 mm.
Population-based criteria for PPD positivity are as follows:
An in vitro blood test based on interferon-gamma release assay (IGRA) with antigens specific for M tuberculosis can also be used to screen for latent TB infection and offers certain advantages over tuberculin skin testing.[36, 37] Currently available tests include QuantiFERON-TB Gold In-Tube (QFT-GIT), an enzyme-linked immunosorbent assay or ELISA based on ESAT-6, CFP-10, and TB 7.7 antigens and T-SPOT.TB, an enzyme-linked immunospot (ELISpot) assay based on ESAT-6 and CFP-10 antigens. Both tests measure in vitro T-cell interferon (IFN)-gamma in response to antigens highly specific for M tuberculosis and absent from the BCG vaccine and M avium.[38]
Overall, sensitivity and specificity of IGRA are comparable to those of tuberculin skin testing; however, unlike tuberculin skin testing, a second encounter for reading is unnecessary. Results are reported as positive, negative, or indeterminate. Patients with an indeterminate result may have evidence of immunosuppression and may be nonreactive on skin testing.[39]
Neither tuberculin skin testing nor IGRA testing is sufficiently sensitive to rule out TB infection.[40] Approximately 20% of patients with active TB, particularly those with advanced disease, may have normal PPD test results.
Limited data exist on the sensitivity of TST and IGRA tests in some situations; caution is recommended on the interpretation of these tests in infants and patients with immunosuppressive conditions.[38]
A systematic review of QuantiFERON-TB Gold (QFT-G)/Gold in-Tube (QFT-G-IT) and T-SPOT.TB by Chang and Leung concluded that QFT-G had the highest positive likelihood ratio (48.1) for latent TB infection and T-SPOT.TB had the best negative likelihood ratio (0.10). A negative T-SPOT.TB result in middle-aged and older patients makes active TB very unlikely.[41]
Results from a study by Leung et al indicated that tuberculin skin testing was not predictive of the subsequent development of active TB.[42] The authors followed 308 males with increased risk of TB due to a diagnosis of silicosis. A positive T-SPOT.TB finding was associated with a relative risk of 4.5 for subsequent TB in the group overall and a relative risk of 8.5 among the men who did not receive preventive treatment for latent TB. CFP-10 spot count was more predictive than the ESAT-6 spot count.
In a separate study by Diel et al, all subjects who developed active tuberculosis within 4 years after exposure to a smear-positive index case had positive results using QuantiFERON-TB Gold in-tube.[43]
In a study of kidney-transplant recipients, isoniazid therapy was given to all patients with a significant TST reaction or risk factors for TB infection. ELISPOT assay was performed on all patients. No patients who were treated with isoniazid developed active TB. Among 71 patients with positive ELISPOT who did not receive isoniazid, 4 (6%) subsequently developed active TB after kidney transplantation.[44]
A systematic review of QuantiFERON-TB Gold (QFT-G)/Gold in-Tube (QFT-G-IT) and T-SPOT.TB by Chang and Leung concluded that, at a 90% certainty threshold, latent TB infection is best diagnosed with QFT-G/QFT-G-IT and best excluded with T-SPOT.TB. Neither test can diagnose TB disease, but T-SPOT.TB can exclude it in middle-aged and older patients.[41]
Advantages to IGRA compared with PPD include the following:
The mechanism of tuberculin skin testing is based on the fact that latent TB infection induces a strong cell-mediated immune response by measuring the delayed-type hypersensitivity response to intradermal inoculation of tuberculin PPD.
The PPD test is given in an intradermal injection of 5 units of purified protein derivative, preferably with a 26-, 27-, or 30-gauge needle. These delayed-type hypersensitivity tests should be read between 48 and 72 hours after administration.
A negative response in immunologically intact individuals measures less than 5 mm.
Population-based criteria for PPD positivity are as follows:
- For patients who are HIV positive, have abnormal chest radiographic findings, have significant immunosuppression, or have had recent contact with persons with active TB, the cutoff is 5 mm or more induration.
- For patients who are intravenous drug users, residents of nursing homes, prisoners, impoverished persons, or members of minority groups, the cutoff is 10 mm or more induration.
- For patients who are young and in good health, the cutoff is 15 mm or more induration.
An in vitro blood test based on interferon-gamma release assay (IGRA) with antigens specific for M tuberculosis can also be used to screen for latent TB infection and offers certain advantages over tuberculin skin testing.[36, 37] Currently available tests include QuantiFERON-TB Gold In-Tube (QFT-GIT), an enzyme-linked immunosorbent assay or ELISA based on ESAT-6, CFP-10, and TB 7.7 antigens and T-SPOT.TB, an enzyme-linked immunospot (ELISpot) assay based on ESAT-6 and CFP-10 antigens. Both tests measure in vitro T-cell interferon (IFN)-gamma in response to antigens highly specific for M tuberculosis and absent from the BCG vaccine and M avium.[38]
Overall, sensitivity and specificity of IGRA are comparable to those of tuberculin skin testing; however, unlike tuberculin skin testing, a second encounter for reading is unnecessary. Results are reported as positive, negative, or indeterminate. Patients with an indeterminate result may have evidence of immunosuppression and may be nonreactive on skin testing.[39]
Neither tuberculin skin testing nor IGRA testing is sufficiently sensitive to rule out TB infection.[40] Approximately 20% of patients with active TB, particularly those with advanced disease, may have normal PPD test results.
Limited data exist on the sensitivity of TST and IGRA tests in some situations; caution is recommended on the interpretation of these tests in infants and patients with immunosuppressive conditions.[38]
A systematic review of QuantiFERON-TB Gold (QFT-G)/Gold in-Tube (QFT-G-IT) and T-SPOT.TB by Chang and Leung concluded that QFT-G had the highest positive likelihood ratio (48.1) for latent TB infection and T-SPOT.TB had the best negative likelihood ratio (0.10). A negative T-SPOT.TB result in middle-aged and older patients makes active TB very unlikely.[41]
Results from a study by Leung et al indicated that tuberculin skin testing was not predictive of the subsequent development of active TB.[42] The authors followed 308 males with increased risk of TB due to a diagnosis of silicosis. A positive T-SPOT.TB finding was associated with a relative risk of 4.5 for subsequent TB in the group overall and a relative risk of 8.5 among the men who did not receive preventive treatment for latent TB. CFP-10 spot count was more predictive than the ESAT-6 spot count.
In a separate study by Diel et al, all subjects who developed active tuberculosis within 4 years after exposure to a smear-positive index case had positive results using QuantiFERON-TB Gold in-tube.[43]
In a study of kidney-transplant recipients, isoniazid therapy was given to all patients with a significant TST reaction or risk factors for TB infection. ELISPOT assay was performed on all patients. No patients who were treated with isoniazid developed active TB. Among 71 patients with positive ELISPOT who did not receive isoniazid, 4 (6%) subsequently developed active TB after kidney transplantation.[44]
A systematic review of QuantiFERON-TB Gold (QFT-G)/Gold in-Tube (QFT-G-IT) and T-SPOT.TB by Chang and Leung concluded that, at a 90% certainty threshold, latent TB infection is best diagnosed with QFT-G/QFT-G-IT and best excluded with T-SPOT.TB. Neither test can diagnose TB disease, but T-SPOT.TB can exclude it in middle-aged and older patients.[41]
Advantages to IGRA compared with PPD include the following:
- One patient visit
- Ex vivo tests
- No booster effect
- Independent of BCG vaccination
- High cost
- More laboratory resources required
- Complicated process of lymphocyte separation
- Lack of prospective studies
ELISpot Testing of Other Fluids
Jafari et al found that an M tuberculosis
–specific ELISpot assay can be used to differentiate TB cases with
sputum smear negative for acid-fast bacteria (AFB) from latent TB
infection. In a prospective study of 347 patients suspected of having
active TB who were unable to produce sputum or who had AFB-negative
sputum smears, ELISpot testing of bronchoalveolar lavage fluid displayed
a sensitivity and specificity of 91% and 80%, respectively, for the
diagnosis of active pulmonary TB.[45]
Additional Rapid Tests
Other
rapid tests are also available, such as BACTEC-460 (Becton-Dickinson),
ligase chain reaction; and luciferase reporter assay (within 48 h)
(Franklin Lakes). These tests have been developed for rapid
drug-susceptibilities testing, which can be available within 10 days.
Drug resistance tests such as the FASTPlaque TB-RIF for rifampin resistance can be used after growth in semiautomated liquid cultures such as BACTEC-460; rifampin resistance can be used as a surrogate marker for isoniazid resistance.
Drug resistance tests such as the FASTPlaque TB-RIF for rifampin resistance can be used after growth in semiautomated liquid cultures such as BACTEC-460; rifampin resistance can be used as a surrogate marker for isoniazid resistance.
Urinalysis
Urinalysis
and urine culture can be obtained for patients with genitourinary
complaints. Patients are often asymptomatic; however, significant pyuria
and/or hematuria with no routine bacterial organisms should prompt
urine culture for acid-fast bacilli.
HIV Testing
All
patients who are diagnosed with active tuberculosis (TB) and who are
not known to be HIV positive should be considered for HIV testing.
This
is the drug of choice for preventive therapy and the primary drug in
combination therapy for active TB. In patients receiving treatment for
active TB, pyridoxine 25-50 mg PO qd should be coadministered to prevent
peripheral neuropathy..
Rifampin
is used in combination with at least 1 other antituberculous drug. It
inhibits DNA-dependent bacterial, but not mammalian, RNA polymerase.
Cross-resistance may occur.
In most susceptible cases, the patient undergoes 6 months of treatment. Treatment lasts for 9 months if the patient's sputum culture result is still positive after 2 months of therapy.
This
is a pyrazine analog of nicotinamide that is either bacteriostatic or
bactericidal against M tuberculosis, depending on the concentration of
drug attained at site of infection. Pyrazinamide's mechanism of action
is unknown.
Administer the drug for the initial 2 months of a 6-month or longer treatment regimen for drug-susceptible TB. Treat drug-resistant TB with individualized regimens.
Ethambutol
diffuses into actively growing mycobacterial cells (eg, tubercle
bacilli). It impairs cell metabolism by inhibiting the synthesis of 1 or
more metabolites, which in turn, causes cell death. No cross-resistance
has been demonstrated.
Mycobacterial resistance is frequent with previous therapy. In such cases, use ethambutol in combination with second-line drugs that have not been previously administered. Administer every 24 hours until permanent bacteriologic conversion and maximal clinical improvement are observed. Absorption is not significantly altered by food.
Streptomycin
sulfate is used for the treatment of susceptible mycobacterial
infections. Use this agent in combination with other antituberculous
drugs (eg, isoniazid, ethambutol, rifampin). The total period of
treatment for TB is a minimum of 6 months. However, streptomycin therapy
is not commonly used for the duration of therapy. The drug is
recommended when less potentially hazardous therapeutic agents are
ineffective or contraindicated.
Levofloxacin,
a second-line drug, is used in combination with rifampin and other
antituberculous agents in TB treatment. Levofloxacin is useful in
treating most cases of MDR-TB.
Moxifloxacin inhibits the A subunits of DNA gyrase, resulting in inhibition of bacterial DNA replication and transcription.
This
agent is used in once-weekly regimens along with isoniazid. Rifapentine
should not be used in individuals with HIV or with positive cultures
after 2 months of treatment.
Ethionamide
is a second-line drug that is bacteriostatic against M tuberculosis. It
is recommended if treatment with first-line drugs (isoniazid, rifampin)
is unsuccessful. Ethionamide can be used to treat any form of active
TB. However, it should be used only with other effective antituberculous
agents.
Amikacin
is a second-line drug that irreversibly binds to the 30S subunit of
bacterial ribosomes. It blocks the recognition step in protein
synthesis, causing growth inhibition. Use the patient's ideal body
weight for dosage calculation.
Cycloserine,
a second-line drug, inhibits cell wall synthesis in susceptible strains
of gram-positive and gram-negative bacteria and in M tuberculosis. It
is a structural analogue of D-alanine, which antagonizes the role of
D-alanine in bacterial cell wall synthesis, inhibiting growth.
Capreomycin
is a second-line drug that is obtained from Streptomyces capreolus for
coadministration with other antituberculous agents in pulmonary
infections caused by capreomycin-susceptible strains of M tuberculosis.
Capreomycin is used only when first-line agents (eg, isoniazid,
rifampin) have been ineffective or cannot be used because of toxicity or
the presence of resistant tubercle bacilli.
This
is an ansamycin antibiotic derived from rifamycin S. Rifabutin inhibits
DNA-dependent RNA polymerase, preventing chain initiation. It is used
for TB treatment in individuals on specific HIV medications, when
rifampin is contraindicated (most protease inhibitors).
Clofazimine
inhibits mycobacterial growth, binding preferentially to mycobacterial
DNA. It has antimicrobial properties, but its mechanism of action is
unknown. Always use this drug with other antituberculous agents.
This
is a bacteriostatic agent that is useful against Mycobacterium
tuberculosis. It inhibits the onset of bacterial resistance to
streptomycin and isoniazid.
Administer aminosalicylate sodium with other antituberculous drugs.
Medication Summary
The goals of pharmacotherapy are to reduce morbidity and to prevent complications.
Treatment of tuberculosis has 3 basic therapeutic principles. First, any regimen must use multiple drugs to which M tuberculosis is susceptible. Second, the therapy must be taken regularly. Third, the therapy must continue for a period sufficient to resolve the illness.
New cases are initially treated with 4 drugs: isoniazid, rifampin, pyrazinamide, and either ethambutol or streptomycin for 2 months; they are then treated with a continuation phase of 4 months with isoniazid and rifampin. Retreatment cases should initially receive at least 5 drugs, including isoniazid, rifampin, and at least 2 new drugs to which the patient has not been exposed.
Treatment of tuberculosis has 3 basic therapeutic principles. First, any regimen must use multiple drugs to which M tuberculosis is susceptible. Second, the therapy must be taken regularly. Third, the therapy must continue for a period sufficient to resolve the illness.
New cases are initially treated with 4 drugs: isoniazid, rifampin, pyrazinamide, and either ethambutol or streptomycin for 2 months; they are then treated with a continuation phase of 4 months with isoniazid and rifampin. Retreatment cases should initially receive at least 5 drugs, including isoniazid, rifampin, and at least 2 new drugs to which the patient has not been exposed.
Antitubercular agents
Class Summary
The goals of tuberculosis (TB) treatment are to shorten the clinical course of TB, prevent complications, prevent the development of latency and/or subsequent recurrences, and decrease the likelihood of TB transmission. In patients with latent TB, the goal of therapy is to prevent progression of disease.Isoniazid
Rifampin (Rifadin)
In most susceptible cases, the patient undergoes 6 months of treatment. Treatment lasts for 9 months if the patient's sputum culture result is still positive after 2 months of therapy.
Pyrazinamide
Administer the drug for the initial 2 months of a 6-month or longer treatment regimen for drug-susceptible TB. Treat drug-resistant TB with individualized regimens.
Ethambutol (Myambutol)
Mycobacterial resistance is frequent with previous therapy. In such cases, use ethambutol in combination with second-line drugs that have not been previously administered. Administer every 24 hours until permanent bacteriologic conversion and maximal clinical improvement are observed. Absorption is not significantly altered by food.
Streptomycin
Levofloxacin (Levaquin)
Moxifloxacin (Avelox)
Rifapentine (Priftin)
Ethionamide (Trecator)
Amikacin
Cycloserine (Seromycin)
Capreomycin (Capastat)
Rifabutin (Mycobutin)
Clofazimine (Lamprene)
Para-aminosalicylic acid (Paser)
Administer aminosalicylate sodium with other antituberculous drugs.
There is a safe & effective Natural Herbal Medicine. For Total Cure Call +2348142454860, or email him drrealakhigbe@gmail.com For an Appointment with (Dr.) AKHIGBE contact him. Treatment with Natural Herbal Cure. Treatment with Natural Herbal Cure For: Painful or Irregular Menstruation. HIV/Aids. Diabetics. Vaginal Infections. Vaginal Discharge. Itching Of the Private Part. Breast Infection. Discharge from Breast. Breast Pain & Itching. Lower Abdominal Pain. No Periods or Periods Suddenly Stop. Women Sexual Problems. Chronic Disease. Pain during Sex inside the Pelvis. Pain during Urination. Pelvic Inflammatory Disease, (PID). Dripping Of Sperm from the Vagina As Well As for Low sperm count. Parkinson disease. Lupus. Cancer. Tuberculosis. Zero sperm count. Asthma. Quick Ejaculation. Premature Ejaculation. Herpes. Joint Pain. Stroke. Weak Erection. Discharge from Penis. STD. Staphylococcus + Gonorrhea + Syphilis. Heart Disease. Pile-Hemorrhoid. Waist & Back Pain. Male Infertility and Female Infertility. Etc. Take Action Now. contact him & Order for your Natural Herbal Medicine: +2348142454860 and email him drrealakhigbe@gmail.com Note For an Appointment with (Dr.) AKHIGBE.I suffered in Cancer for a year and three months dieing in pain and full of heart break. One day I was searching through the internet and I came across a testimony herpes cure by doctor Akhigbe. So I contact him to try my luck, we talk and he send me the medicine through courier service and with instructions on how to be drinking it.To my greatest surprise drinking the herbal medicine within three weeks I got the changes and I was cure totally. I don't really know how it happen but there is power in Dr Akhigbe herbal medicine. He is a good herbalist doctor.
ReplyDelete